● International-standard quality
● Localization advantages in China
● Professional eCOA provider
01
02
03
04
05
06
07
08
09

Source:《Patient non-compliance with paper diaries》Arthur A Stone, Saul Shiffman, Joseph E Schwartz, Joan E Broderick, Michael R Hufford
In Compliance With the Internationally Accepted ALCOA+ Data Quality Standards ALCOA+ Data Quality Standards are a set of principles widely applied to ensure the quality and reliability of data collected in clinical trials.
The DCT SaaS platform is a visual low-code platform for clinical study designers.
Sponsors can opt for self-service, full-service, or hybrid modes based on project needs. Our goal is to offer transparent, convenient digital solutions for eCOA in clinical research, enhancing trial efficiency while optimizing costs.

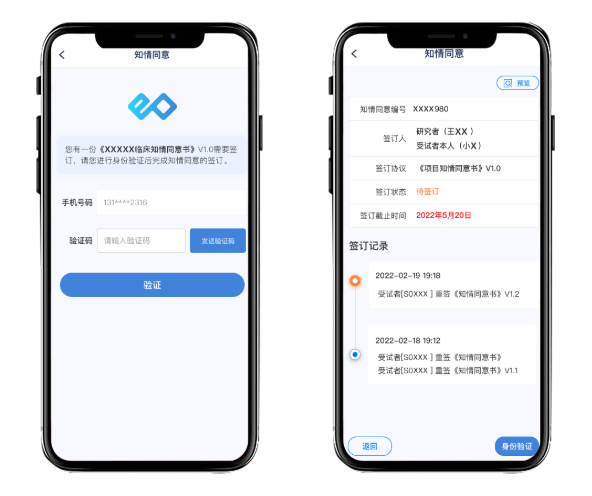
By utilizing multimedia and remote methods, patients can more easily and fully understand the content of the informed consent, which not only improves the patient experience but also practices the "patient-centered" drug development philosophy.
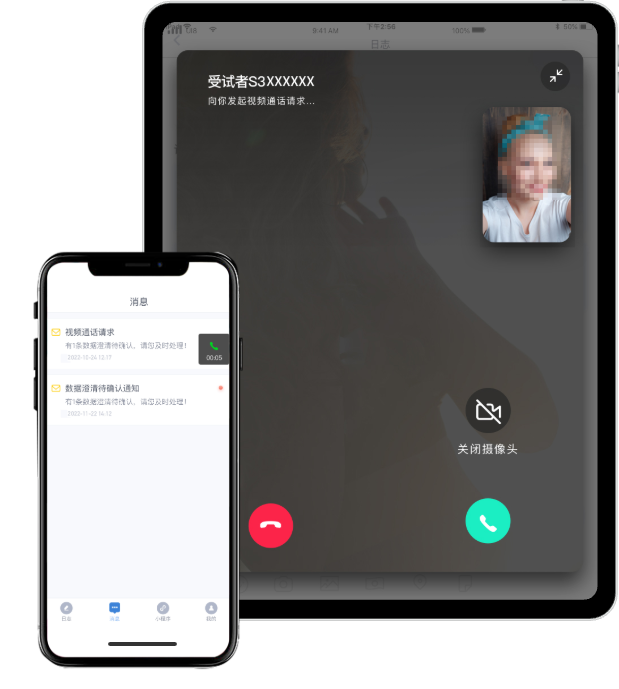
In clinical research centered around the "patient-centered" approach, appropriate consideration should be given to adopting remote visit modes. By providing remote and online participation options, more patients can be given the opportunity to participate in clinical studies. Without the constraints of time and location, this approach enhances the patient experience, reduces the burden on patients, and enriches the diversity of clinical study sample sizes.
Wearable devices, as an important component of DCT (Decentralized/Digitalized Clinical Trials), not only enable real-time collection of patients' physiological information but can also be integrated into eCOA (electronic Clinical Outcome Assessment) applications to provide convenience for patients. When monitoring and reporting safety events, wearable devices can promptly transmit the collected data to researchers, ensuring patient safety.

One-click universal account access, without additional burden
Mobile app operation with stable system and convenient operation.
Real-time data access, keeping information at your fingertips.

One-click universal access for administrator accounts, without any additional burden.
The administrator end can manage patient health data.
Check real-time details of patient data (such as electrocardiograms, etc.).
Track device usage for convenient management.